Transcatheter Mitral Valve Repair (TMVr)
As one of the highest volume and most experienced transcatheter mitral valve programs in the nation, Northwestern Medicine continues to expand its Transcatheter Heart Valve Program by offering a wide array of minimally invasive commercially available and clinical trial options to treat the mitral valve, including MitraClip.
Transcatheter Mitral Heart Valve Therapies: MitraClip, Now Helping More Patients
The Transcatheter Heart Valve Program at Northwestern Medicine offers a wide variety of commercially available (FDA approved) and clinical trial options for the mitral valve, allowing us to tailor treatment precisely to each patient’s individual needs. For some patients with mitral valve disease, transcatheter heart valve therapies, like MitraClip, are an alternative treatment to open heart valve surgery.
In 2013, the U.S. FDA approved MitraClip for patients with degenerative or primary mitral regurgitation caused by disease of the mitral valve. In 2019, results from the COAPT™ clinical trial results1 showed that treatment with MitraClip leads to a reduction in hospitalizations and death compared to medical therapy alone.
| COAPTTM Clinical Trial Results1: MitraClip vs. Medical Therapy in Advanced Heart Failure Patients with Secondary or Functional Mitral Regurgitation |
||
| MitraClip + Medical Therapy |
Medical Therapy Alone |
|
| Annualized HF hospitalizations |
35.8% (lower number is better) |
67.9% |
| All-Cause Mortality (death) |
29.1% (lower number is better) |
46.1% |
| Alive and Moderately Improved Quality of Life2 |
36.4% (higher number is better) |
16.6% |
| Alive and Substantially Improved Quality of Life2 |
29.1% (higher number is better) |
11.7% |
As a result of these findings, the FDA approved MitraClip for patients with functional or secondary mitral regurgitation caused by diminished heart function. This approval has significantly expanded the number of people who can be treated with MitraClip.
As a leader in transcatheter mitral valve treatment options, Northwestern Memorial Hospital not only has one of the highest-volume MitraClip programs in Illinois3, but is also active in training physicians across the country on this and other advanced techniques.
Transcatheter Mitral Valve Commercially Available Treatment Options
Transcatheter procedures allow the procedure to be performed while the patient’s heart is still beating, eliminating the need for a “heart bypass” machine and its associated risks. Due to the minimally invasive nature of transcatheter procedures, patients tend to recover faster and experience an improvement in symptoms soon after the procedure is completed. The Transcatheter Heart Valve Program at Northwestern Medicine offers two mitral valve transcatheter treatment options: MitraClip and transcatheter mitral valve in valve replacement.
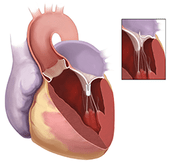
MitraClip: A minimally invasive treatment option for patients with mitral regurgitation. In patients with mitral regurgitation, the mitral valve does not close completely, allowing blood to flow backward or “leak,” creating symptoms that may include shortness of breath, fatigue and chest pain. MitraClip is the only device currently approved by the FDA for transcatheter mitral valve replacement (TMVR).
During MitraClip implantation, an interventional cardiologist inserts a catheter (tube) in the body through the femoral (groin) vein. The MitraClip device is compressed and advanced by a catheter through the vein until it reaches the diseased mitral valve. Once in the heart, the MitraClip device is positioned to join or “clip” together a portion of the mitral valve, reducing or eliminating the backward flow of blood.
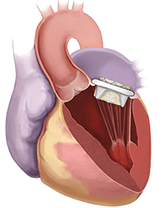
Transcatheter Mitral Valve in Valve Replacement: A minimally invasive TMVR procedure for patients who have had previous open heart valve surgery to replace the mitral valve with a bioprosthetic/tissue valve and the valve is now failing. Instead of the failing mitral valve being replaced during another open heart valve surgery, the failing mitral valve is replaced by placing a TMVR valve inside the failing mitral valve.
During mitral valve in valve replacement, an interventional cardiologist and/or a cardiac surgeon inserts a catheter (tube) through a vein in the body. The TMVR valve is compressed and advanced by a catheter through a vein until it reaches the failing mitral valve. A balloon on the catheter expands and secures the TMVR valve within the failing mitral valve. The catheter is then removed and the secured TMVR valve remains in place pushing the failing mitral tissue valve out of the way, allowing the TMVR valve to take over the job of regulating blood flow from the heart.
Transcatheter Mitral Valve Clinical Trial Options
Researchers at Northwestern Medicine conduct clinical trials that provide access to innovative therapies for the treatment of heart and vascular disease. Participating in a clinical trial is an opportunity to evaluate the effectiveness and safety of medications or study devices. The study devices and delivery systems used in the following clinical trials are investigational (experimental), which means they are not approved for commercial use by the U.S. FDA.
APOLLO: enrolling patients to evaluate the Intrepid Transcatheter Mitral Valve Replacement (TMVR) System (study device) in people with moderate-to-severe or severe, symptomatic mitral regurgitation who may not be optimally treated with approved transcatheter repair or surgical mitral valve intervention. Eligible participants will receive a TMVR using the Intrepid valve.
CardioMech MVRS EFS: The purpose of this early feasibility study is to evaluate the CardioMech Mitral Valve Repair System (MVRS) in patients diagnosed with moderate to severe (≥3+) or severe (≥4+), symptomatic, degenerative mitral regurgitation and who are determined to be at intermediate or high surgical risk for mitral valve repair. The CardioMech MVRS is designed to reduce the amount of mitral regurgitation without the need for surgery (less invasive). Up to 25 eligible participants will receive the investigational valve and be followed for 5 years.
TWIST EFS Early Feasibility Study is enrolling adult patients with symptomatic moderate-severe and severe, mitral regurgitation (MR) who are at elevated risk for surgical mitral valve repair or replacement. Eligible participants will receive the INNOVALVE Transseptal Mitral Valve Replacement (TMVR) System (study device) which is designed to reduce the amount of mitral regurgitation without the need for surgery (less invasive).
Northwestern Medicine is also among the most experienced programs in the country for transcatheter treatment options for the aortic valve and tricuspid valve.
Meet the Transcatheter Mitral Valve Therapies Team
| Northwestern Medicine Bluhm Cardiovascular Institute is a nationally recognized destination for those who require highly specialized cardiovascular care. | Meet the Teams Downtown Chicago Western Suburbs |
1. Health Status After Transcatheter Mitral Valve Repair in Patients with Heart Failure and Secondary Mitral Regurgitation: COAPT Trial. JACC, 2019.
2. Health status evaluated at baseline, 1, 6, 12, and 24 months using the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the Medical Outcomes Study Short Form 36 (SF-36) Health Survey.
3. IL IP COMPdata FY16 October-FY18 June YTD, last accessed December 2018.
Northwestern Medicine Transcatheter Mitral Featured Publications:
- Panchal HB, Stone GW, Saxena A, Bursac Z, Veledar E, Nagabandi A, Davidson CJ, Leon MB, Beohar N. In-hospital outcomes after transcatheter edge-to-edge mitral valve repair in patients with chronic kidney disease: An analysis from the 2010-2016 National inpatient sample. Catheter Cardiovasc Interv. 2021 Nov 15;98(6):1177-1184. doi: 10.1002/ccd.29712. Epub 2021 Apr 15. PMID: 33856107.
- Lim DS, Smith RL, Zahr F, Dhoble A, Laham R, Lazkani M, Kodali S, Kliger C, Hermiller J, Vora A, Sarembock IJ, Gray W, Kapadia S, Greenbaum A, Rassi A, Lee D, Chhatriwalla A, Shah P, Rodés-Cabau J, Ibrahim H, Satler L, Herrmann HC, Mahoney P, Davidson C, Petrossian G, Guerrero M, Koulogiannis K, Marcoff L, Gillam L; CLASP IID Pivotal Trial Investigators. Early outcomes from the CLASP IID trial roll-in cohort for prohibitive risk patients with degenerative mitral regurgitation. Catheter Cardiovasc Interv. 2021 Oct;98(4):E637-E646. doi: 10.1002/ccd.29749. Epub 2021 May 18. PMID: 34004077.
- Kislitsina ON, Zareba KM, Bonow RO, Andrei AC, Kruse J, Puthumana J, Akhter N, Malaisrie SC, McCarthy PM, Rigolin VH. Is mitral valve disease treated differently in men and women? Eur J Prev Cardiol. 2019 Sep;26(13):1433-1443. doi: 10.1177/2047487319833307. Epub 2019 Mar 4. PMID: 30832507.
- McCarthy PM, Davidson CJ, Kruse J, Lerner DJ, Braid-Forbes MJ, McCrea MM, Elmouelhi AM, Ferguson MA. Prevalence of atrial fibrillation before cardiac surgery and factors associated with concomitant ablation. J Thorac Cardiovasc Surg. 2020 Jun;159(6):2245-2253.e15. doi: 10.1016/j.jtcvs.2019.06.062. Epub 2019 Jul 10. PMID: 31444073.
- Desai A, Thomas JD, Bonow RO, Kruse J, Andrei AC, Cox JL, McCarthy PM. Asymptomatic degenerative mitral regurgitation repair: Validating guidelines for early intervention. J Thorac Cardiovasc Surg. 2021 Mar;161(3):981-994.e5.
- Mack MJ, Abraham WT, Lindenfeld J, Bolling SF, Feldman TE, Grayburn PA, Kapadia SR, McCarthy PM, Lim DS, Udelson JE, Zile MR, Gammie JS, Gillinov AM, Glower DD, Heimansohn DA, Suri RM, Ellis JT, Shu Y, Kar S, Weissman NJ, Stone GW. Cardiovascular Outcomes Assessment of the MitraClip in Patients with Heart Failure and Secondary Mitral Regurgitation: Design and rationale of the COAPT trial. Am Heart J. 2018 Nov;205:1-11. doi: 10.1016/j.ahj.2018.07.021. Epub 2018 Aug 1. PMID: 30134187.
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021 Feb 2;77(4):e25-e197. doi: 10.1016/j.jacc.2020.11.018. Epub 2020 Dec 17. Erratum in: J Am Coll Cardiol. 2021 Feb 2;77(4):509. Erratum in: J Am Coll Cardiol. 2021 Mar 9;77(9):1275. PMID: 33342586.
*Bolding of author’s name indicates Northwestern Medicine and/or Northwestern University Feinberg School of Medicine faculty.
