First-of-Its-Kind Device Aims to Prevent Sudden Cardiac Death
Northwestern Medicine studies a possible breakthrough in technology to treat cardiac arrest.
Published June 2023
After his second cardiac event in five years, Joseph Mulligan was not surprised when his Northwestern Medicine cardiologist, James D. Flaherty, MD, recommended implanting a device to help keep him alive if he were to have sudden cardiac arrest. At 84 years old, the resident of South Bend, Indiana, knew many people with pacemakers and expected that he, like his friends, would need one, too.
Instead, Dr. Flaherty recommended an implantable cardioverter defibrillator (ICD). Defibrillators and pacemakers are different:
The EV ICD is a revolution in implantable defibrillator technology.— Bradley P. Knight, MD
- A pacemaker uses painless electrical impulses to treat slow heart rates.
- A defibrillator applies a shock to the heart to treat very fast heart rhythms.
Worldwide, hundreds of thousands of defibrillators are implanted in patients every year to treat dangerously fast heart rhythms. Traditional, or transvenous (within the heart), ICDs have thin wires (leads) threaded directly into the heart and veins. Traditional ICDs are under constant stress from the heart beating and movement of the upper body.
An alternate option is a defibrillator that is placed under the skin, called a subcutaneous defibrillator. However, this kind of device is very large and cannot pace the heart; it only restarts the heart if it stops.
Pivotal Study Leads to More Treatment Options
Joseph’s Northwestern Medicine care team presented him with a unique option: join a clinical trial to test the safety and efficacy of a first-of-its-kind extravascular (outside the heart), implantable cardioverter defibrillator (EV ICD). The Medtronic EV ICD is designed to treat dangerously fast heart rhythms that can lead to sudden cardiac arrest, while avoiding certain risks of traditional ICDs.
The goal of the EV ICD system is to provide the benefits of traditional ICDs, but with:
- A smaller device
- Greater device longevity
- No leads in the heart or surrounding vessels
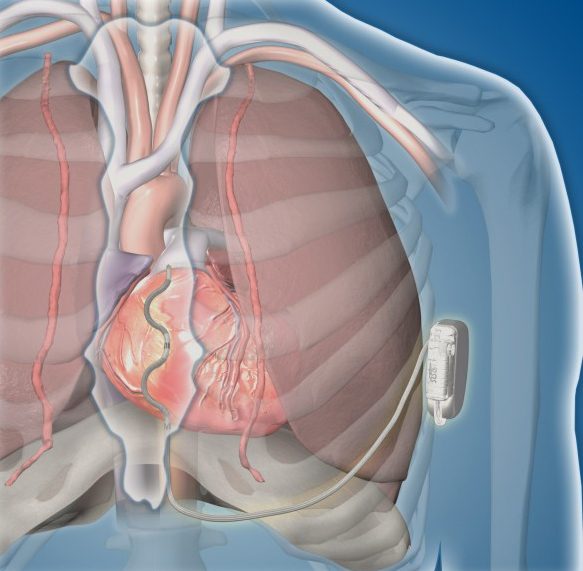
“By positioning the leads outside of the heart, there is less risk of long-term complications associated with leads in the heart and veins,” says Bradley P. Knight, MD, medical director of Electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute and a co-author of the study.
Joseph was one of 356 participants enrolled in the EV ICD clinical trial in 17 countries across North America, Europe, the Middle East, Asia, Australia and New Zealand. Participants received the same therapies provided by traditional ICDs, including defibrillation, anti-tachycardia pacing and back-up pacing therapies, with this single implanted device.
Bluhm Cardiovascular Institute is the only site in Chicago and one of only 46 sites worldwide to participate in the pivotal research.
The Results
Results of the clinical trial were published in The New England Journal of Medicine. The trial showed:
- Defibrillation therapy effectiveness at implant was 98.7%, which is greater than the defibrillation effectiveness of traditional transvenous ICDs.
- At six months, 92.6% of patients were free from major system and/or procedure-related complications such as hospitalization, system revision or death.
“The EV ICD is a revolution in implantable defibrillator technology that addresses the limitations of our existing therapies that have been available for several decades,” says Dr. Knight. “With the promising data from this trial, we have an opportunity to evolve ICD technology in a safe, effective manner and improve this lifesaving therapy.”
For Joseph, now 86 years old, the option to enroll in the clinical trial is one he is thankful for as he continues to lead a very active life with the EV ICD as a safety net.
“I feel terrific, and I’m grateful to have the advantage of having this device, which I know is keeping me alive longer than I should be,” says Joseph. “The whole process was very easy. I was only hospitalized overnight, and my scar has already almost completely disappeared.”
To learn more about cardiovascular clinical trials at Northwestern Medicine, visit the Bluhm Cardiovascular Institute – Clinical Trials Unit.
Worldwide, the EV ICD system is investigational and not yet approved for sale or distribution. Medtronic has received FDA approval for a Continued Access Study, and patients in the study will continue to be followed until Medtronic receives regulatory approval for the EV ICD system in the United States. The clinical trial was funded by Medtronic; ClinicalTrials.gov number NCT04060680.